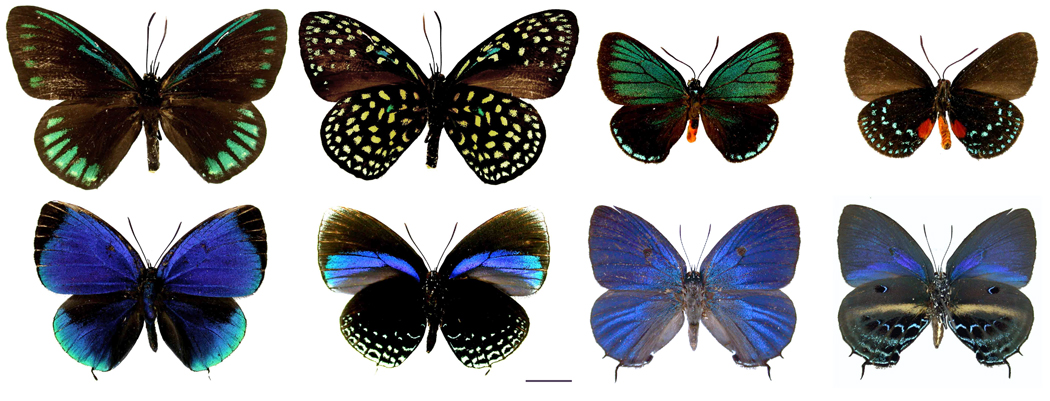
Eumaeus atala butterfly (top right) and Eumaeus childrenae (top left) with other close relatives studied by the research team, including Theorema eumenia (bottom left) and Mithras nautes (bottom right); all shown from above on the
Scientists Discover How a Group of Caterpillars Became Poisonous
The Atala butterfly (Eumaeus atala) and its five closest relatives in the genus Eumaeus like to display their toxicity. This sextet’s toxicity comes from what they eat as caterpillars: plants called cycads that have been around since before dinosaurs roamed the Earth and contain a potent liver toxin called cycasin.
Because they are filled with poison, Eumaeus are big, gaudily iridescent and flap about like they have no place to go. Even their caterpillars are conspicuous, congregating in groups to munch cycad plants all while sporting flashy red and gold coloration. Their ostentatious qualities all signal to predators that they are a not a good meal; in nature, being toxic protects organisms from being attacked if their predators know it.
Now, new research led by the Smithsonian’s National Museum of Natural History butterfly curator Bob Robbins tells the evolutionary tale of how these six poisonous butterflies gained their toxin-laced defenses as well as the bold colors and behaviors that tell all would-be predators to steer clear.
“Butterflies don’t have teeth or claws to defend themselves,” Robbins said. “But they use their wing color and flight behavior as a signal of their unsavory qualities to predators, sometimes deceptively and in others truthfully, as in the case of Eumaeus. With the newfound ability to sequence genomes with relative ease, we have the opportunity to look at that in great detail for the first time.”
The paper, published in the Feb. 8 issue of the journal the Proceedings of the National Academy of Sciences, relies on 46 sequenced butterfly genomes, including all six members of Eumaeus and a number of members of the 1,000-strong group of butterflies Eumaeus is related to—the hairstreaks.
Hairstreaks occur widely in North and South America, and almost all of them are small, evasive and nondescript. Their caterpillars, which typically eat a varied diet of flowering plants, usually are well camouflaged and do not congregate in groups. Eumaeus’ ranks are notable exceptions; so much so that for years some researchers suggested that Eumaeus might not even be hairstreaks at all. In addition, because of cycads’ ancient evolutionary history, most scientists had assumed that the six members of Eumaeus developed their tolerance for cycasin and their conspicuousness a very long time ago in their evolutionary history.
To settle the matter, Robbins and his colleagues set about sequencing the genomes of Eumaeus’ six members and a bevy of other hairstreaks around three years ago, drawing on the wealth of diverse specimens held in the museum’s collections as well as some samples from wild butterflies. Those results definitively showed that Eumaeus butterflies were not an ancient evolutionary departure from the rest of the hairstreaks. In fact, they are closely related to a pair of rather typical genera called Theorema and Mithras.
The results also showed that the ability to eat poisonous cycads was such a boon to Eumaeus that it spurred a frenzy of rapid evolutionary change that outpaced all other hairstreaks. The team also learned that Eumaeus had split in two evolutionary lines after they began to eat cycads, so they were able to analyze the evolutionary dash twice, including the marked genetic similarities the two lineages had in responding to their new poisonous diet.
When the team started analyzing the Eumaeus genomes, they saw a striking amount of genetic change to parts of these butterflies’ genomes related to building various types of proteins. To narrow down whether these proteins might be related to eating cycads, the researchers compared the Eumaeus genomes to butterflies in Theorema, their closest non-toxic relatives, which have camouflaged, solitary caterpillars that eat a standard range of flowering plants.
Theorema also had some regions of fast evolving DNA that coded the construction of various proteins. When Robbins and his co-authors compared the two genomes they discarded any regions of rapid change that overlapped between the two to hopefully isolate the genetic changes involved in coping with the cycad’s toxins.
“Sure enough, there was a core group of proteins that had gone through a lot of rapid change in Eumaeus but not in the hairstreaks that don’t eat cycads,” Robbins said. “When we looked at the function of the proteins that changed quickly, it was very strong on the proteins that would destroy cells, proteins that would remove dead cell debris and proteins to create new cells.”
Robbins said these proteins are exactly what an organism would need if it had to find a way to safely ingest cycasin-type toxin. “If the cycad toxins were killing cells at high rates, the organism would need to break those cells down, clean them out and then very rapidly make new ones to avoid any ill effects,” he said.
This research details the evolution of a toxic defense mechanism in these six butterfly species and the genetic consequences that result. Though the emergence of warning coloration and lazy flight might not be as easy to pick out in Eumaeus’ genes, it appeared in parallel with the change in diet.
Numerous butterfly species have evolved to make themselves less attractive to predators by eating toxic substances, and Robbins said this evolutionary accounting of how Eumaeus honed its defense could offer an evolutionary model for how species adapt to living with these toxins inside their bodies.
Funding and support for this research were provided by the Smithsonian.
SI-37-2021